 Many companies produce the foods we eat. Do you ever wonder why or how they test for salt during the production process?
Many companies produce the foods we eat. Do you ever wonder why or how they test for salt during the production process?
Examples by Manufacturing Type
Frozen Vegetable Processor
|
The salt content of the blanching water is important for maintaining the bright colors of vegetables
|
A Condiment Manufacturer
|
Testing Sauces and dressings
|
A Cheese Maker
|
Measure the salinity of saltwater that the cheese is soaked in
|
A Potato Chips Manufacturer
|
Checking for salt sprinkled on fried potato slices
|
A fresh Cut Fruit Processor
|
Use a 2% saline solution with a small amount of ascorbic acid to prevent discoloration of fruits
|
A Deli Food Supplier
|
Measure foods with a salt meter vs. by taste
|
A Canned Food Manufacturing Plant
|
Measure the brine for canned tuna
|
A Pickles Manufacturer
|
Measure the salinity of the brine for salt-packed products
|
A Cold Cut Meat Manufacturer
|
Measure salt concentration of ham and deli slices
|
A Baker
|
Measure and monitor the salinity of bread dough to around 1%-2%
|
Salt which is made up of 40% sodium and 60% Chloride is an important ingredient found in food. While salt can make food taste better, control color, and maintain food texture, it is also considered a health-risk factor (mostly due to the sodium). Measuring and controlling the levels of salt between the extremes is a constant battle. Producers of processed foods generally have the biggest need for identifying and controlling salt levels to address not only the taste, color, and texture of foods but also to address some of the healthier eating lifestyles more and more consumers are demanding.
For these reasons it is paramount that salt is measured accurately. So how do we do that?
Food comes in a variety of forms. Solid, Liquids, pastes, creams, pieces, chunks, wafers, crackers, gooey, sauces, liquids with chunks in them...let's see what else..Anyway, you get the idea. There are a lot of ways food can be produced and consumed!
So what device or devices can we use to measure the salt found in these numerous forms of processed foods?
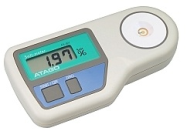
Well, there are a number of "salt meters" out there that can measure salt. However, not all salt meters can measure the particular salt you are looking for in the same way. In fact some "salt meters" can only measure salt under certain conditions and or in certain substances like water or sea water. For this reason it is important to first consider what your going to be testing. For example, If your food sample includes "food stuff particles" that you can grind into a paste form, then you can probably use a salt meter that utilizes the conductivity method. On the other hand if you have a brine that you immerse food into and your only concerned with the liquid then perhaps a different salt meter will work.
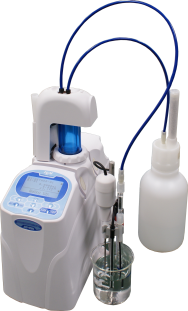
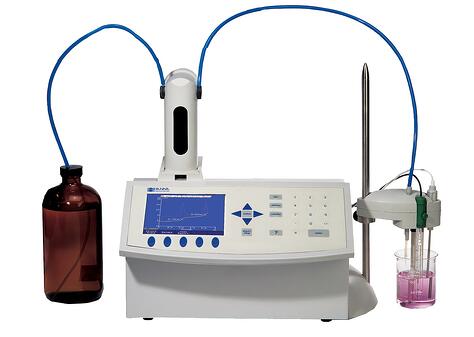
The point is this. The form of the food at the instant you are going to perform the test is key. Many types of foods can be formed into pastes and diluted with water. If the food you need to test is like this then a simple salt meter utilizing the conductivity method may be able to perform the test to your satisfaction. I say may because % salt levels and other accuracy factors may require that you use an entirely different method of titration known as silver nitrate titration instead.
A brief explanation and description of the two measurement approaches:
The Mohr method, also known as a silver nitrate titration method, utilizes the characteristics of silver nitrate that reacts with chloride ions to measure the salinity %.

Conversley, some of the more popular salt meters emloy the electric conductivity method. Both methods measure the salinity but operate on different measurement principles. However, by creating a conversion table between the two testing methods, correlation between the set of results can be seen.

Aside from the measurement capabilities of each approach there are pros and cons to each.
Pro's and Con's
|
|
Salt Meter
|
Titration
|
Measurement Range
|
Less..maybe
|
More
|
Accuracy
|
Less
|
More
|
Ease of Use
|
More
|
Less
|
Time to test
|
Less
|
More
|
Cost
|
Less
|
More
|
While each method has benefits we have recently found through some informal surveying that some food processors are choosing to use both methods. These companies are finding that it is easier to use the hand held devices and perform quick spot checks on the production line. If any problems are identified on the production line then further verification and testing can be performed using the titration approach. Some think using this collaborative approach is ideal.
ALSO READ OUR MOST RECENT UPDATES TO THIS BLOG POST : Salt related posts








 Many companies produce the foods we eat. Do you ever wonder why or how they test for salt during the production process?
Many companies produce the foods we eat. Do you ever wonder why or how they test for salt during the production process?



 using a Volumetric Karl Fischer Titrator tend to have difficulty in 3 areas. Unlike
using a Volumetric Karl Fischer Titrator tend to have difficulty in 3 areas. Unlike 
 In this presentation we discuss the basic Karl Fischer Water Standards and talk about some of their uses for both Coulometric and Volumetric Karl Fischer Titration. We also describe some of the related problems that can be identified and overcome by using Karl Fischer Water Standards.
In this presentation we discuss the basic Karl Fischer Water Standards and talk about some of their uses for both Coulometric and Volumetric Karl Fischer Titration. We also describe some of the related problems that can be identified and overcome by using Karl Fischer Water Standards.
 As a service provider of Karl Fisher testing apparatus, we see different moisture testing issues that many operators, managers, and even companies face. We have come to realize that helping operators become more knowledgeable about "the little things" can help boost confidence, improve performance and efficiency, and ensure accurate testing.
As a service provider of Karl Fisher testing apparatus, we see different moisture testing issues that many operators, managers, and even companies face. We have come to realize that helping operators become more knowledgeable about "the little things" can help boost confidence, improve performance and efficiency, and ensure accurate testing. 
 There are multiple methods of moisture determination, including loss on drying, Karl Fischer titration, piezoelectric sorption, spectroscopy, and chilled mirrors among others. However, it is advantageous to use Karl Fischer (KF) titration in moisture analysis for the following reasons:
There are multiple methods of moisture determination, including loss on drying, Karl Fischer titration, piezoelectric sorption, spectroscopy, and chilled mirrors among others. However, it is advantageous to use Karl Fischer (KF) titration in moisture analysis for the following reasons: Karl Fischer titrator. So let's get started. There are two things to consider. First, you have the chemical limitations of the reagents themselves. Second, you have the user/operator variable. Sometimes changing the reagent has more to do with the condition of the reagent sitting in the vessel. How full is the vessel after running numerous test? How long has the reagent been sitting in the vessel? How messy is the reagent and sample residue inside the vessel? Sometimes the user may simply want to replace the reagents because they look dirty/messy or their vessel is too full from adding samples during previous tests.
Karl Fischer titrator. So let's get started. There are two things to consider. First, you have the chemical limitations of the reagents themselves. Second, you have the user/operator variable. Sometimes changing the reagent has more to do with the condition of the reagent sitting in the vessel. How full is the vessel after running numerous test? How long has the reagent been sitting in the vessel? How messy is the reagent and sample residue inside the vessel? Sometimes the user may simply want to replace the reagents because they look dirty/messy or their vessel is too full from adding samples during previous tests. What most operators are attempting to do is determine whether their Karl Fisher titrator is measuring moisture accurately.
What most operators are attempting to do is determine whether their Karl Fisher titrator is measuring moisture accurately.
