WHY DO WE CARE ABOUT BROMINE NUMBER AND BROMINE INDEX ANYWAY?
The degree of unsaturated hydrogen carbide (Hydrocarbon) in oil and petroleum products is indicated by Bromine number or index. Hydrocarbons containing between 6 and 10 carbon molecules are the primary components of most fuels where the process of burning them produces energy. Saturated hydrocarbons are defined by molecular structure and are defined as Alkanes. Alkanes are the basis for petroleum fuels. Unsaturated hydrocarbons are also defined by their molecular structure and produce less energy when burned than do saturated hydrocarbons. Mathematically it therefore requires a greater quantity of unsaturated hydrocarbons to be burned to equal the same amount of energy produced by burning the same amount of saturated hydrocarbons. An un-friendly environmental side effect of burning hydrocarbons is the creation of carbon dioxide. So from an environmental standpoint you could say we prefer the lesser of two evils and prefer to burn saturated hydrocarbons vs. unsaturated hydrocarbons. For these reasons alone it would make sense that we would want to measure and quantify these hydrocarbons.
HYDROCARBONS AND SOME OF THEIR USES
| NAME |
USES |
| Methane |
Fuel in electrical generation |
| Ethane |
Chemical applications |
| Propane |
Heating and cooking |
| Butane |
lighters and aerosol |
| Pentane |
Lab & production of polystyrene |
| Hexane |
Glue |
| Heptane & Octane |
Major contributor to gasoline |
| Nonane |
Diesel fuel
|
|
Decane
|
Jet fuel, gasoline and diesel
|
Although the test method ASTM2710 describes the use of an automatic potentiometric titrator the coulometric Karl Fischer method is easier to perform (see ASTMD1492-08e1).
<BROMINE NUMBER AND INDEX BY COULOMETRIC KARL FISCHER TITRATION>
Bromine number :The amount of bromine (unit: g/100) consumed in 100g sample.
Bromine index :The amount of bromine (unit: mg/100) consumed in 100g sample.
<PRINCIPAL OF MEASUREMENT>
The unsaturated hydrogen carbon reacts with bromine as follows: R-CH=CH-R+Br2→R-CHBr-CHBr-R- (Eq. #1)
In coulometric titration, bromine is generated by electrolysis of anolyte containing bromine ion: 2Br-Br2+2e- (Eq. #2)
When generated bromine is consumed according to Eq. (#1), the electrode detects bromine consumption, and continues generating bromine according to Eq. (#2).
Such bromine is generated in proportion to the electricity determined by Faraday’s Law. From Eq. (#1), Bromine reacts with coupled C=C evenly (1:1). ==>Thus, one mol of bromine(159.8g) is equivalent to 2 96500 coulomb, which means 1.2 coulomb/1mgBr2.
Based on the above principle, the electricity consumed in electrolysis is converted to the exiting bromine.
Special notes to obtain correct measurement results:
- Replace anolyte and catholyte reagent often (daily).
- When anolyte turns to white color replace it with new anolyte.
- Over use of anolyte and catholyte and not replacing it can cause results to be higher than expected.
- Do not share Bromine titration cell with a moisture titration cell

Step 1: Preparation of Reagents
Prepare the reagents and samples for measurement of bromine number and bromine index.
Use the following reagent for measurement of bromine number or index:
Anolyte Reagent: Mixture of Acetic acid (high grade) 600mL; Methanol (high grade) 260mL; 1M-Potassium bromide solution 140mL. Blend it well and use 100mL each time.
Catholyte Reagent: 0.2M-Potassium chloride solution. Use 5mL each time.
Check Solution: 0.05Wt-Cyclohexene toluene mixture (Approx. 93 ~ 102mgBr2/100g). Theoretical value is calculated by the below formula:
Step 2: Prepare Reagent for testing
Refer to the below chart for sample size:
| Bromine index (mg/100g) |
Sample Size (g) |
|
Below 10
10 ~ 50
50 ~100
100 ~ 200
More than 200
|
10 ~ 15
5 ~ 10
3 ~ 5
1 ~ 3
~ 1
|
For bromine number, a sample is diluted with toluene to Bromine number 0.2 (g/100g), and approximately 1g is used for measurement. Calculate the toluene to be used for diluting to Bromine number 0.2 (g/100g) with the following equation:

Obtain the dilution coefficient in advance according to the following equation:
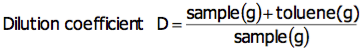
Step 3: Measurement Procedure
Measure Bromine Number and Bromine Index
-
Discharge 20 100μL check solution into the electrolysis cell, and press [Pre-titr.*] key.
-
After pre-titration is finished, press [Sample*] key to enter sample name, its ID and size, and enter the dilution coefficient (D) .
Press [MENU/HOME*] to Main display.
-
Press [Start] key, and discharge the sample into the cell. Again press [Start*] key for titration to start.
-
After titration is over, the measurement results appear on display with printout when a printer is connected.
*Note that different Karl Fischer Titrator Models will have different button commands. The MKC-710 series coulometric Karl Fischer titrators come standard with pre-programmed setup for running Bromine and follow the above button commands.
We hope this information helps you get started. If you have any questions or would like some help please contact us by clicking on the button below.




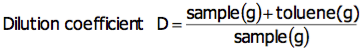
 Don’t know whether you need to run a Bromine Number or Bromine Index? Not sure what the difference is between Electrometric or Coulometric? And just how many approved ASTM methods are there, anyway?
Don’t know whether you need to run a Bromine Number or Bromine Index? Not sure what the difference is between Electrometric or Coulometric? And just how many approved ASTM methods are there, anyway? 


