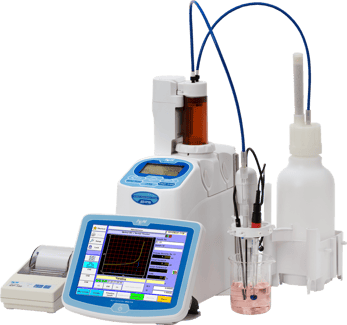
Recently, we worked with a customer who owns one of our Volumetric Karl Fischer Titrators (MKV-710) and was experiencing unexpected issues when testing samples with high moisture content. Specifically, the customer observed that certain samples appeared to max out at around 1% moisture, after which the titrator would go into over-titration. Interestingly, this behavior only occurred with specific sample types.
To get to the root of the issue, we stepped back and reviewed the fundamentals of volumetric Karl Fischer titration. This back-to-basics approach helped clarify what was happening—and revealed several practical troubleshooting steps that may help others facing similar challenges.
Understanding the Basics
The MKV-710 Volumetric Karl Fischer Titrator is designed to measure moisture content from trace levels all the way up to 100% water, so measuring above 1% moisture should not be a limitation of the instrument itself.
Reagent Factor Check
The customer provided data showing that the Karl Fischer reagent factor was properly calculated using Honeywell Hydranal Composite 5, with a factor of:
5.0796 mg/mL
This is a very reasonable value and indicates the reagent and factor calculation were done correctly. Great start.
What Causes Over-Titration?
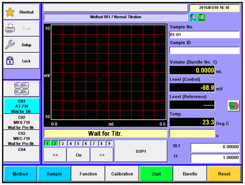
Over-titration occurs when the instrument delivers more Karl Fischer reagent than is required for the amount of water actually present in the titration cell.
Here’s how the process works:
-
The detector electrode senses moisture in the titration vessel.
-
It signals the titrator to dose Karl Fischer reagent (Composite 5).
-
The reagent reacts with and neutralizes the water.
-
Once all moisture is consumed, the electrode stops detecting water.
-
The instrument then calculates total water based on how much reagent was used.
If the electrode receives inaccurate information, the titrator may continue dosing reagent—leading to over-titration.
The Math Behind the Measurement
Understanding the math helps clarify what the instrument is doing:
-
Composite 5 reagent neutralizes approximately 5 mg of water per mL
-
1 mg = 1,000 micrograms
-
Therefore, 1 mL of reagent ≈ 5,000 micrograms of water
-
All Karl Fischer titrators measure moisture in micrograms
Key Conversions
-
10,000 ppm = 1.0% moisture
-
5,000 ppm = 0.5% moisture
-
1,000 ppm = 0.1% moisture
Moisture Calculation Formula
Moisture (PPM or %) = Water detected (µg) / Sample size (g)
As long as the sample size is known, the moisture result can be accurately calculated from the amount of reagent consumed.
Common Causes of Over-Titration
If the instrument is overdosing reagent, a few common issues may be responsible:
1. Detector Electrode Issues
A faulty or aging detector electrode may not correctly sense when all moisture has been neutralized, sending incorrect signals to the titrator.
2. Poor Sample Mixing (Very Common)
If the sample does not dissolve or homogenize properly in the titration solvent, moisture can exist in localized “pockets.” This can confuse the detector electrode and cause continued dosing.
Solution:
Improve sample mixing and solubility. In some cases, adding a co-solvent such as xylene can significantly help break down the sample.
Pro Tip: Xylene can be added at up to 20% of the total sample volume to improve solubility.
Additional Pro Tips for Reliable Results
-
Verify Instrument Performance
Run a water check using a micro-drop of pure water. A recovery of 99% or higher confirms the titrator can accurately measure high moisture levels. When combined with a successful factor check, this strongly indicates the instrument is working correctly. -
Increase Pre-Stirring Time
If samples have solubility challenges, adjust the method to stir for 60 seconds before titration begins. This simple change can dramatically improve consistency. -
Use Mixed Solvents When Needed
For stubborn samples, try a solvent blend such as 80% methanol / 20% xylene to improve dissolution. -
Watch for Chemical Interferences
Samples containing ketones, aldehydes, or problematic pH levels can interfere with Karl Fischer reactions and should be evaluated carefully.
Final Thoughts
When high-moisture samples appear to “max out” or cause over-titration, the issue is often sample-related rather than instrument-related. Verifying the basics—reagent factor, instrument performance, electrode condition, and sample mixing—can quickly narrow down the root cause.
A systematic troubleshooting approach not only resolves the immediate problem but also builds confidence in the results moving forward.
















 A well-known company called Honeywell purchased and now controls the Hydranal line of Karl Fischer Reagents. You can still order Hydranal brand
A well-known company called Honeywell purchased and now controls the Hydranal line of Karl Fischer Reagents. You can still order Hydranal brand 
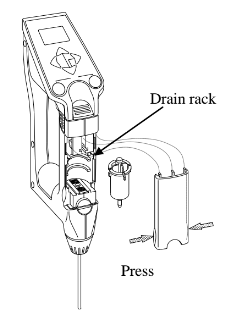

 Consistometer and are familiar with it's operation. Pressing down the gate and loading the trigger is step one. Pouring in your sample and scraping off the top to a clean and even surface is important to getting repeatable results. With a stopwatch in one hand, "popping" the trigger and letting the sample flow out and down the trough comes next. Some tests are designed to see how far a sample will flow in say, 10 seconds. Another testing approach might be to time the movement of the sample until it reaches a pre-determined point (bostwick). If you have ever noticed in the bottom of the tray there are lines with numbers ranging from 0 to 24 (you really can't see a number 24 because the tray stops exactly at what would be 24). Over the years these lines with numbers have affectionatly become to be known as "bostwicks".
Consistometer and are familiar with it's operation. Pressing down the gate and loading the trigger is step one. Pouring in your sample and scraping off the top to a clean and even surface is important to getting repeatable results. With a stopwatch in one hand, "popping" the trigger and letting the sample flow out and down the trough comes next. Some tests are designed to see how far a sample will flow in say, 10 seconds. Another testing approach might be to time the movement of the sample until it reaches a pre-determined point (bostwick). If you have ever noticed in the bottom of the tray there are lines with numbers ranging from 0 to 24 (you really can't see a number 24 because the tray stops exactly at what would be 24). Over the years these lines with numbers have affectionatly become to be known as "bostwicks". 

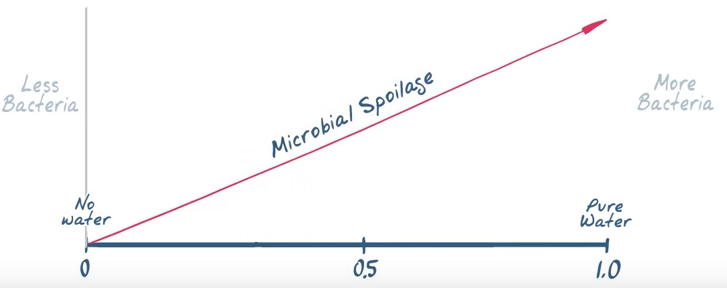
 thermodynamic measure of water expressed as the vapor pressure of water in a sample divided by vapor pressure of pure water at a given temperature.
thermodynamic measure of water expressed as the vapor pressure of water in a sample divided by vapor pressure of pure water at a given temperature. 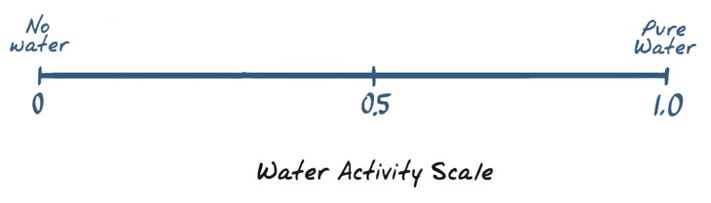





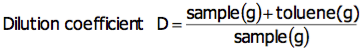












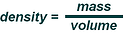
 >What is the mass of the liquid?,
>What is the mass of the liquid?,
 Petroleum is used mostly by volume for the production of fuel, gasoline and
Petroleum is used mostly by volume for the production of fuel, gasoline and

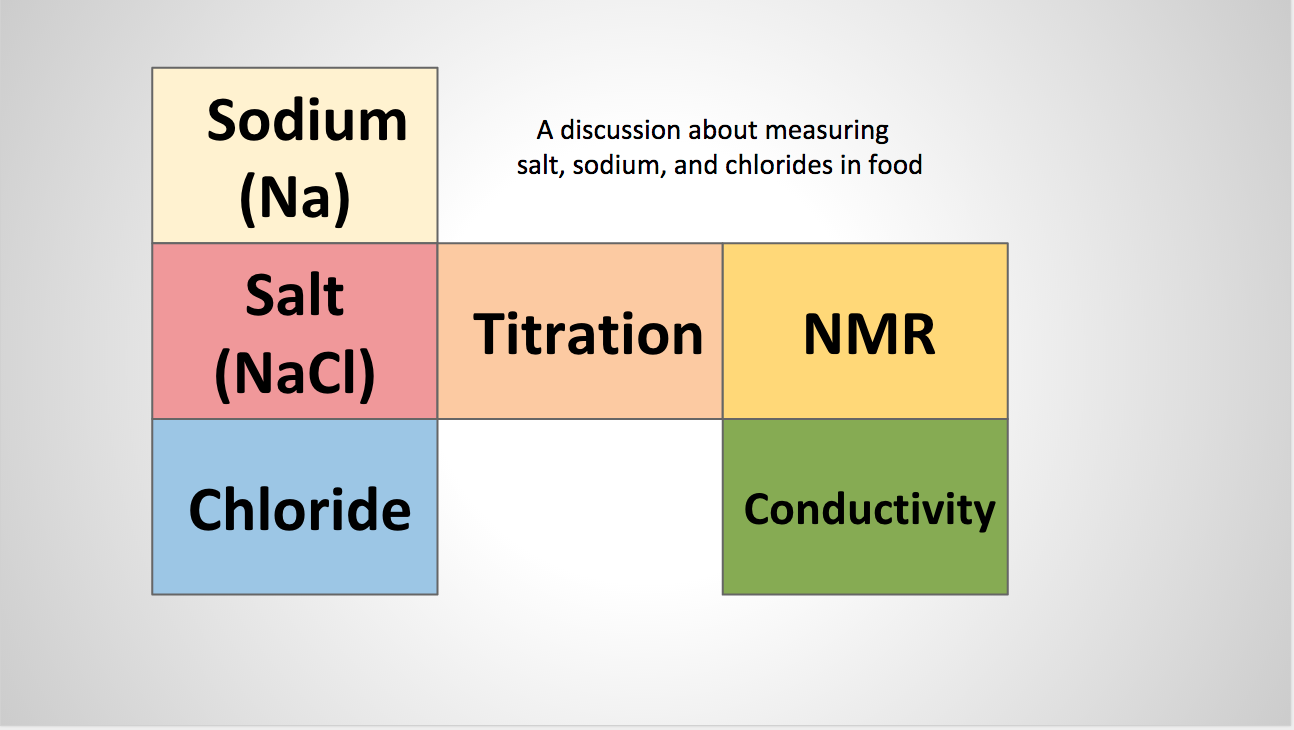


 with the more well known negative effects we associate with sodium (Na) and high blood pressure.
with the more well known negative effects we associate with sodium (Na) and high blood pressure. 

 Certain types of Karl Fischer vessels require the use of Karl Fischer grease. Vessels with smooth port openings need a thin layer of the grease applied to plugs, electrodes, dessicant tubes, bubbler tubes and injection port plugs to help form a snug fit. Decreasing or limiting "ambient moisture" from getting into the vessel - otherwise known as "drift" - is the key benefit of using Karl Fischer grease. Another benefit of Karl Fischer grease is that it also helps prevent chipping of glass on glass fittings. Watch this short video to see how Karl Fischer Grease should be applied.
Certain types of Karl Fischer vessels require the use of Karl Fischer grease. Vessels with smooth port openings need a thin layer of the grease applied to plugs, electrodes, dessicant tubes, bubbler tubes and injection port plugs to help form a snug fit. Decreasing or limiting "ambient moisture" from getting into the vessel - otherwise known as "drift" - is the key benefit of using Karl Fischer grease. Another benefit of Karl Fischer grease is that it also helps prevent chipping of glass on glass fittings. Watch this short video to see how Karl Fischer Grease should be applied. What are the side-effects?
What are the side-effects? 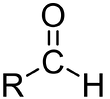








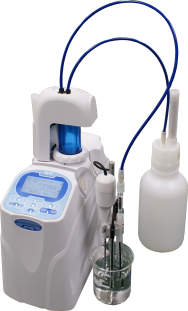
 integrated mini sample changer. The mini sample changer can hold up to 6 samples. The design utilizes a rotating arm that holds the electrode(s), dispensing nozzle(s), and propeller stirrer above each sample and maneuvers from sample to sample. The compact design allows a small footprint on the bench because the titrator sits on top of the sample changer. Watch the short demo video to learn more about this titration system.
integrated mini sample changer. The mini sample changer can hold up to 6 samples. The design utilizes a rotating arm that holds the electrode(s), dispensing nozzle(s), and propeller stirrer above each sample and maneuvers from sample to sample. The compact design allows a small footprint on the bench because the titrator sits on top of the sample changer. Watch the short demo video to learn more about this titration system.
 Don’t know whether you need to run a Bromine Number or Bromine Index? Not sure what the difference is between Electrometric or Coulometric? And just how many approved ASTM methods are there, anyway?
Don’t know whether you need to run a Bromine Number or Bromine Index? Not sure what the difference is between Electrometric or Coulometric? And just how many approved ASTM methods are there, anyway? 


 Most of us know about the Heat Index (HI) and might have even seen a sling psychrometer used by a coach or trainer. How heat measurements were taken before tended to limit the amount of measurements taken due to the separate actions required to determine humidity levels and related temperature readings along with other factors. A confusing process. In many cases measurements were done before and after a practice session - and that was it.
Most of us know about the Heat Index (HI) and might have even seen a sling psychrometer used by a coach or trainer. How heat measurements were taken before tended to limit the amount of measurements taken due to the separate actions required to determine humidity levels and related temperature readings along with other factors. A confusing process. In many cases measurements were done before and after a practice session - and that was it.



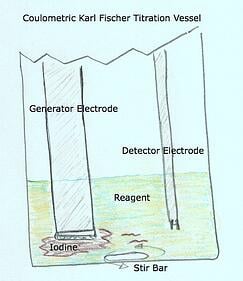

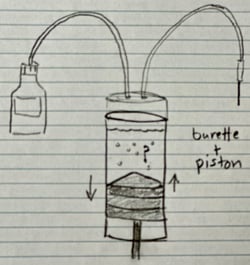
 In a volumetric system the reagent setup is different where a composite or titrant is introduced via a burette piston through a titration nozzle. The amount of composite or titrant delivered is based upon the commands of the titrator. The command from the titrator to the burette and piston that push out the “iodine” through the titration nozzle is, yes, given by the detector electrode. For the purposes of this discussion the difference between the coulometric and volumetric setup is that the delivery of iodine is different. But the same problem can occur where the iodine does not mix well and therefore trick the detector electrode in to thinking there is not enough iodine present inside the vessel to counter and neutralize the water. Since both coulometric and volumetric Karl Fischer Titrators use detector electrodes the problems mentioned earlier about the detector electrode will hold true with volumetric titrators also.
In a volumetric system the reagent setup is different where a composite or titrant is introduced via a burette piston through a titration nozzle. The amount of composite or titrant delivered is based upon the commands of the titrator. The command from the titrator to the burette and piston that push out the “iodine” through the titration nozzle is, yes, given by the detector electrode. For the purposes of this discussion the difference between the coulometric and volumetric setup is that the delivery of iodine is different. But the same problem can occur where the iodine does not mix well and therefore trick the detector electrode in to thinking there is not enough iodine present inside the vessel to counter and neutralize the water. Since both coulometric and volumetric Karl Fischer Titrators use detector electrodes the problems mentioned earlier about the detector electrode will hold true with volumetric titrators also.  electrode from the vessel all the time?
electrode from the vessel all the time?









 If your operating a dual-reagent
If your operating a dual-reagent 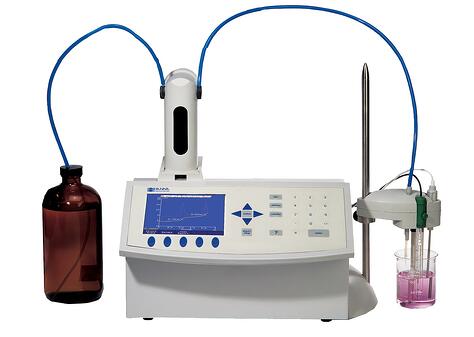


 Many companies produce the foods we eat. Do you ever wonder why or how they test for salt during the production process?
Many companies produce the foods we eat. Do you ever wonder why or how they test for salt during the production process?







 encounter many of the day-to-day problems associated with broken electrodes. In many instances some of the electrodes are destroyed beyond repair but in other circumstances we find that some are in fact capable of being repaired.
encounter many of the day-to-day problems associated with broken electrodes. In many instances some of the electrodes are destroyed beyond repair but in other circumstances we find that some are in fact capable of being repaired. 
 On July 5th, 2008, a workplace rule designed to protect workers from outdoor heat exposure took effect in Washington State. This rule was passed on June 4 after six public hearings were conducted on heat stress and its causes. The hearings confirmed what officials already knew: working outside in hot weather is a health hazard. The three requirements for employers with employees who work outside are to:
On July 5th, 2008, a workplace rule designed to protect workers from outdoor heat exposure took effect in Washington State. This rule was passed on June 4 after six public hearings were conducted on heat stress and its causes. The hearings confirmed what officials already knew: working outside in hot weather is a health hazard. The three requirements for employers with employees who work outside are to: using a Volumetric Karl Fischer Titrator tend to have difficulty in 3 areas. Unlike
using a Volumetric Karl Fischer Titrator tend to have difficulty in 3 areas. Unlike 
 In this presentation we discuss the basic Karl Fischer Water Standards and talk about some of their uses for both Coulometric and Volumetric Karl Fischer Titration. We also describe some of the related problems that can be identified and overcome by using Karl Fischer Water Standards.
In this presentation we discuss the basic Karl Fischer Water Standards and talk about some of their uses for both Coulometric and Volumetric Karl Fischer Titration. We also describe some of the related problems that can be identified and overcome by using Karl Fischer Water Standards.
 As a service provider of Karl Fisher testing apparatus, we see different moisture testing issues that many operators, managers, and even companies face. We have come to realize that helping operators become more knowledgeable about "the little things" can help boost confidence, improve performance and efficiency, and ensure accurate testing.
As a service provider of Karl Fisher testing apparatus, we see different moisture testing issues that many operators, managers, and even companies face. We have come to realize that helping operators become more knowledgeable about "the little things" can help boost confidence, improve performance and efficiency, and ensure accurate testing. 
 Simply put, drift is background moisture that the
Simply put, drift is background moisture that the 
 There are multiple methods of moisture determination, including loss on drying, Karl Fischer titration, piezoelectric sorption, spectroscopy, and chilled mirrors among others. However, it is advantageous to use Karl Fischer (KF) titration in moisture analysis for the following reasons:
There are multiple methods of moisture determination, including loss on drying, Karl Fischer titration, piezoelectric sorption, spectroscopy, and chilled mirrors among others. However, it is advantageous to use Karl Fischer (KF) titration in moisture analysis for the following reasons:
 Karl Fischer titrator. So let's get started. There are two things to consider. First, you have the chemical limitations of the reagents themselves. Second, you have the user/operator variable. Sometimes changing the reagent has more to do with the condition of the reagent sitting in the vessel. How full is the vessel after running numerous test? How long has the reagent been sitting in the vessel? How messy is the reagent and sample residue inside the vessel? Sometimes the user may simply want to replace the reagents because they look dirty/messy or their vessel is too full from adding samples during previous tests.
Karl Fischer titrator. So let's get started. There are two things to consider. First, you have the chemical limitations of the reagents themselves. Second, you have the user/operator variable. Sometimes changing the reagent has more to do with the condition of the reagent sitting in the vessel. How full is the vessel after running numerous test? How long has the reagent been sitting in the vessel? How messy is the reagent and sample residue inside the vessel? Sometimes the user may simply want to replace the reagents because they look dirty/messy or their vessel is too full from adding samples during previous tests. What most operators are attempting to do is determine whether their Karl Fisher titrator is measuring moisture accurately.
What most operators are attempting to do is determine whether their Karl Fisher titrator is measuring moisture accurately.

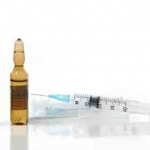 A Word About Karl Fischer Water Standards
A Word About Karl Fischer Water Standards
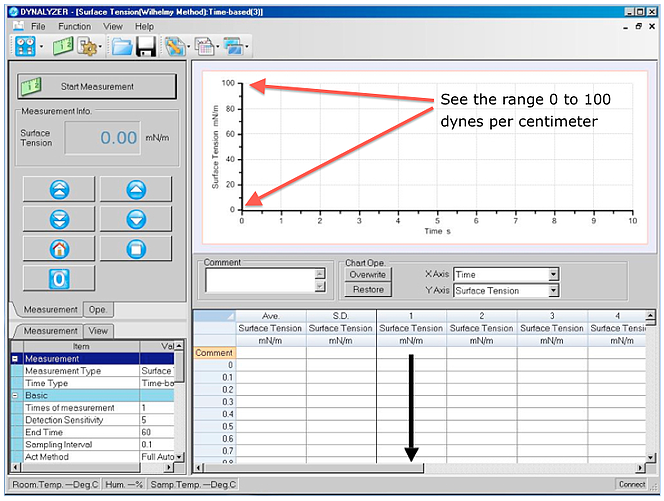
 We all know that surface tension affects our daily lives thru everyday applications like the ink you use in a pen, detergents for washing clothes, soap to clean your hands, paint for the house, just to name a few. But Surface tension is more involved in your life than you may think.
We all know that surface tension affects our daily lives thru everyday applications like the ink you use in a pen, detergents for washing clothes, soap to clean your hands, paint for the house, just to name a few. But Surface tension is more involved in your life than you may think. Most people know about Karl Fisher as a method for determining moisture content. After that there seems to be confusion when the words “Coulometric” and “Volumetric” are mentioned. It goes something like this:
Most people know about Karl Fisher as a method for determining moisture content. After that there seems to be confusion when the words “Coulometric” and “Volumetric” are mentioned. It goes something like this: