This article is not intended to explain a manual titration process but rather how you can configure an automatic titration system to run both acid and salt titrations independently and or as a combined method.
Performing acid and salt titrations is a popular requirement in the food industry. Some foods like tomatoes for example tend to have naturally occurring acidic properties but also take on a salt component when processed into other intermediary products like pizza sauce or spaghetti sauce.
Although sodium (Na) is an important element to measure and report on food labels, salt (NaCl) content is also important in measuring to ensure the taste is good and repeatable during the production process.
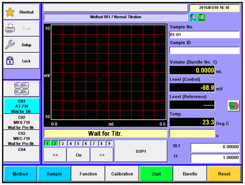
Performing titrations whether manually or with the use of an automatic titrator involves a burette where specific amounts of titrant are delivered to evaluate the potential and or content of what is being measured. Results are usually reported in % for both acidity and salt content.
Here is an example of how you might set up a titration for testing both acidity and salt. Below we have 2 scenarios. The first scenario is configured so that the titration for both acidity and salt can be performed using a single sample. To accomplish this you will need 3 burettes. We will first perform the acidity titration using burette #1 with NaOH (Sodium Hydroxide) as our titrant. In our example, during the acidity titration, the pH will rise to about 8.2. At the end of the acidity titration, the pH level will be too high for us to run the salt titration so we will need to lower the pH. We accomplish this task by dosing HNO3 (Nitric Acid) into the sample using the burette on the Automatic Piston Burette (APB ~ we will call this burette #3). We will dose HNO3 to reduce the pH down to about 4.1. Once the pH level is reduced the salt titration can begin. The second burette (burette #2) located on the titrator then performs the salt titration using silver nitrate (AgNO3) as the titrant. It is worth noting that silver nitrate comes in various strengths and so depending on your sample and the amount of "salt" you expect to find, you may need to adjust the strength (1.0N vs. 0.5N vs 0.1N, etc).
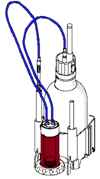
What the setup will look like
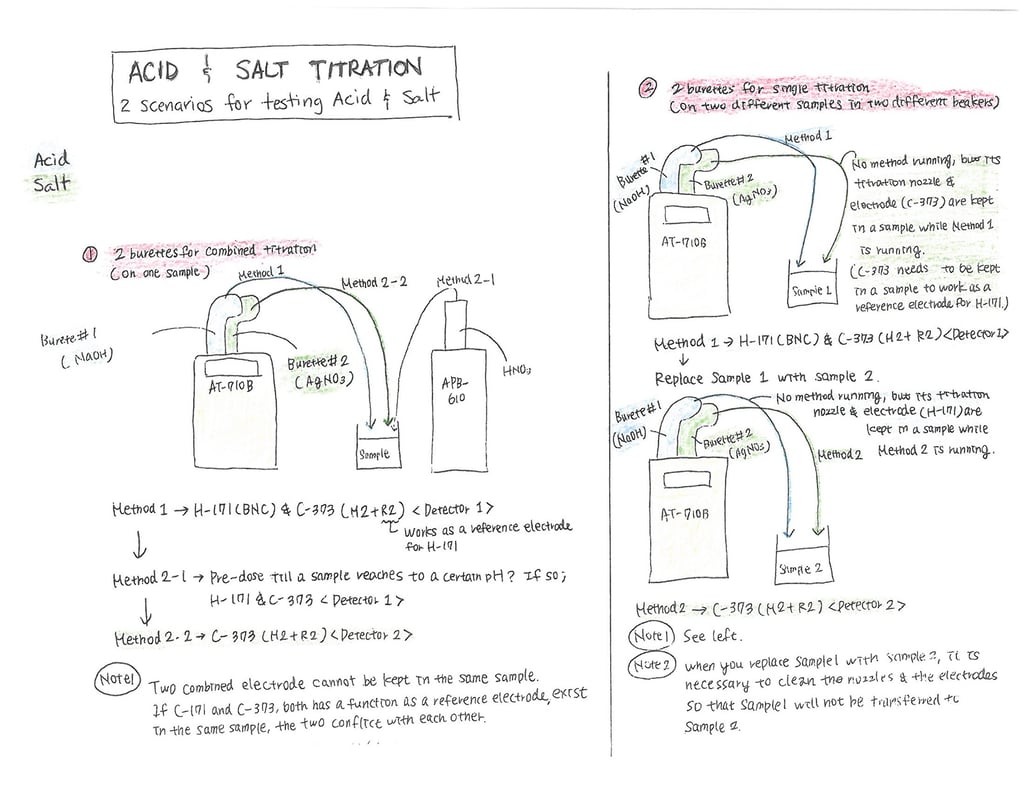
Electrodes we will use:
- pH glass electrode (noted as H171 in the diagram)
- combined silver electrode (noted as C373 in the diagram)
When running the titration using only one sample to obtain both the acidity % and the salt %, we will use both electrodes as the combined silver electrode will act as a reference electrode for the pH electrode.
When running a single titration on two different samples in two different beakers, leave both electrodes and nozzles in the samples ensuring to clean the nozzles and electrodes between tests of each sample. In this scenario, the combined silver electrode (C373) will also work as a reference electrode for the pH electrode (H171) while running the acidity titration.
Summary of key consumable:
- Silver Nitrate (AgNO3) titrant for salt titrations
- Sodium Hydroxide (NaOH) titrant for acidity titrations
- Nitric Acid (HNO3) buffer if combining methods
- Combined Silver Electrode (C373) for salt titrations
- pH glass electrode (H171) for acidity titrations
In the video below we show the titration setup described above.
We hope you find this information useful!






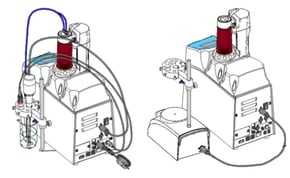

 integrated mini sample changer. The mini sample changer can hold up to 6 samples. The design utilizes a rotating arm that holds the electrode(s), dispensing nozzle(s), and propeller stirrer above each sample and maneuvers from sample to sample. The compact design allows a small footprint on the bench because the titrator sits on top of the sample changer. Watch the short demo video to learn more about this titration system.
integrated mini sample changer. The mini sample changer can hold up to 6 samples. The design utilizes a rotating arm that holds the electrode(s), dispensing nozzle(s), and propeller stirrer above each sample and maneuvers from sample to sample. The compact design allows a small footprint on the bench because the titrator sits on top of the sample changer. Watch the short demo video to learn more about this titration system.