This is a quick guide to getting started and running your first test. We thought it would be helpful for those getting started and checking their first calibration before running tests with actual samples. We will also continue to explore and share more information about calibration options, general maintenance, and data export methods using the DA-130N portable density meter.
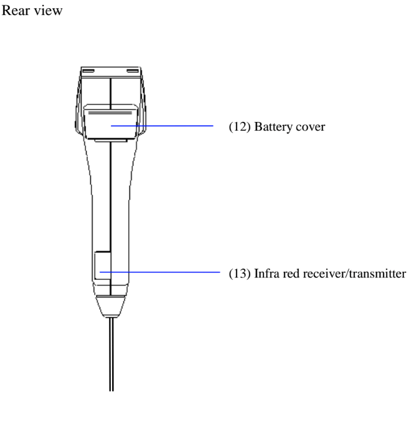
O.K. Let's install three 'AAA' batteries to get started.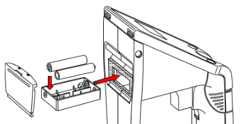
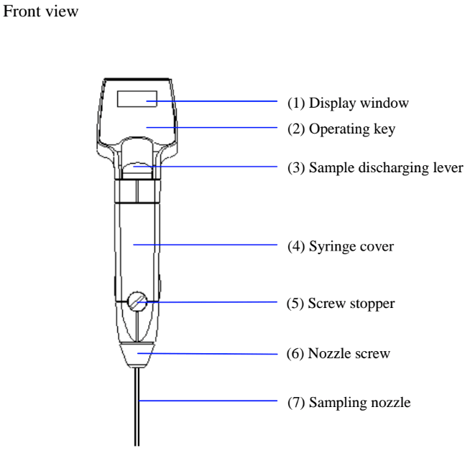
And then attach the sampling nozzle. 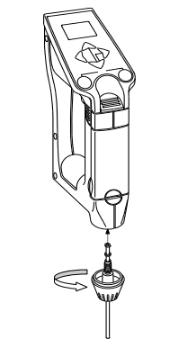
A few words about precautions when taking test measurements.
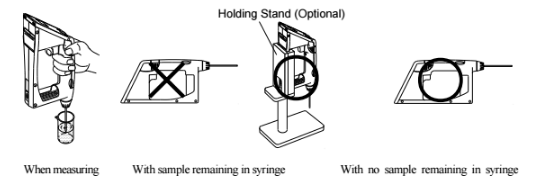
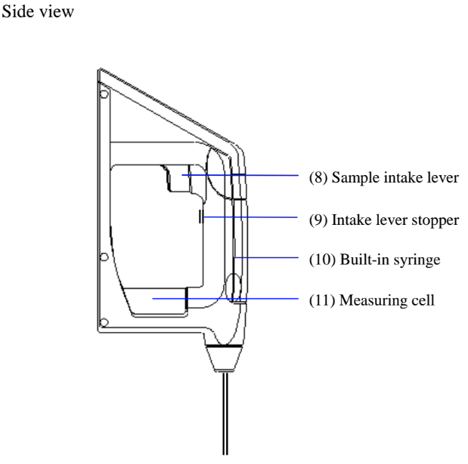
When measuring a sample make sure to hold the instrument so that the sampling nozzle is vertical to the ground. When laying down the instrument between or after measurements make sure to drain the remaining sample from the built-in syring (the built-in syringe is located inside the meter right above the sampling nozzle).
Now let's run a preliminary test using water.
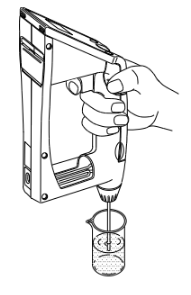
Leave the temperature unit (Celsius), measurement unit (g/cm3) and mode (Field) as initial values. Sample pure water using the built-in syringe. After the measurement cell is filled with sample liquid make sure there are no air bubbles in the cell. If no air bubbles are visible then let's proceed.
Example procedure for running first test.
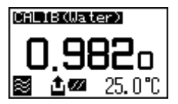
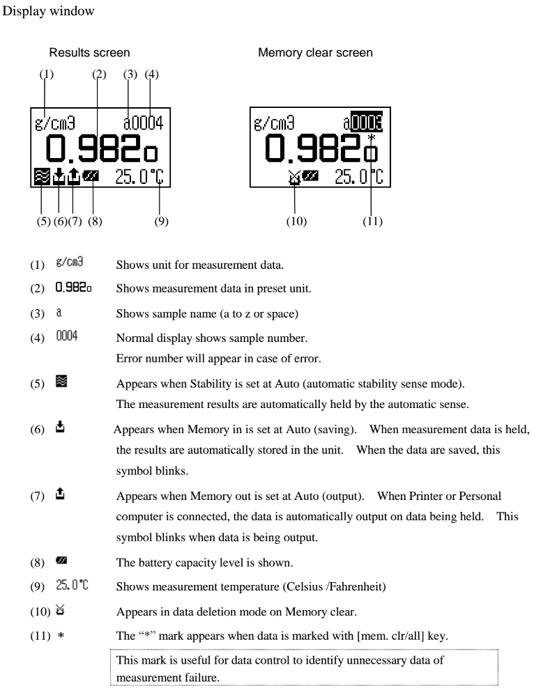
The display changes as above and after confirming it is in the automatic stability sense mode. Press [Meas.] key. When the displayed density is stabalized the value will automatically change reversed as below. Read the data at this point as well as the temperature degree.
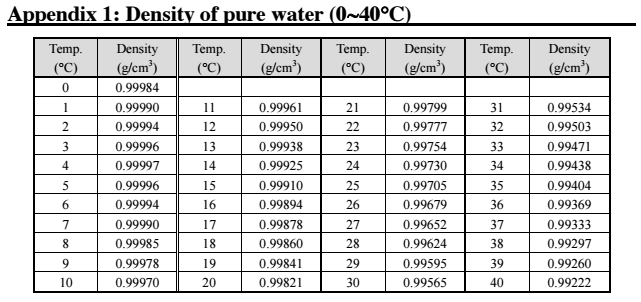
Look up to the pure water density table (appendix 1 in the manual). If the results agree within +-0.001g/cm3, you can proceed to making your sample measurements!
Note: If the results are not within the acceptable range then the measuring cell requires calibration.
Calibration of the measuring cell using pure water
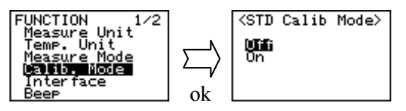
Set calibration mode to OFF. Press the [esc] key 2 times to return to the main menu. Sample pure water making sure no air bubbles are visible. The measuring cell with pure water can be calibrated automatically with the key entries as follows: Press [cal.] key for more than 2 seconds to show CALIB(Water) (inversed colo on the display) showing calibration with pure water in progress.
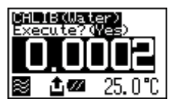
After calibration is over the deviation from the theoretical value will be shown so that you can check if the calibration has been successfully completed.
Note: if after attempting re-calibration of the measuring cell and results are still not within specifications it is possible the measuring cell itself is contaminated. In this case ensure to carefully clean the measuring cell and then re-calibrate again.
Note 1: You can also calibrate with a standard liquid that has a known density. Sometimes calibrating with a liquid that has a similar density to your test samples will help improve precision.
Note 2: You can also calibrate with air.
General maintenance - How to clean and dry the measuring cell and replace the built-in syringe
Step 1: Clean the measuring cell
You can thoroughly clean the measuring cell with just pure water. You can also improve the cleaning of the measuring cell by using Ethyl Alcohol. Ethyl Alcohol gets rid of dirt that cannot be removed with pure water and also reduces the amount of time to dry the measuring cell.
- Estimate 5 to 10 minutes for the measuring cell to dry after cleaning with pure water
- Estimate 3 to 5 minutes for the measuring cell to dry after cleaning with Ethyl Alcoho
Step 2: Purge air to dry the cell thoroughly and completely
option using an air pump:
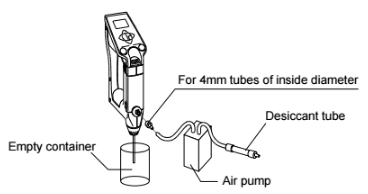
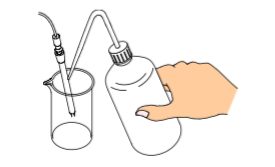
OR use the optional manual pump to dry the cell:
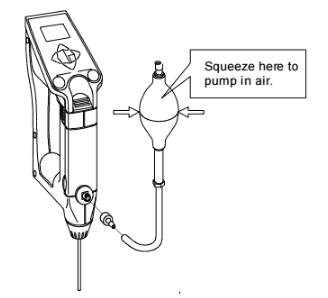
When using the optional manual pump to dry the cell make sure to clean the cell first and then press the sample discharging lever all the way down, and push the sample intake lever upward to lock it. With the adapter for the syringe attached connect the kit as illustrated above and feed air forcefully using the manual bulb.
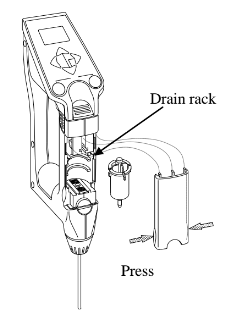
How to replace the built-in syringe.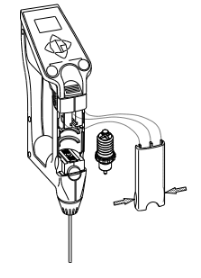
- Press down the sample discharging lever all the way and remove the syringe cover by pulling it toward you as illustrated.
- Turn the syringe 45degrees counter clockwise to release it from the o-ring.
- Slightly pull the sample drain-out lever toward you to pull out the syringe from the o-ring.
- Remove the syringe from the drain rack and replace it with a new one.
- Pull down the drain rack and insert the o-ring and turn it back clockwise until it stops.
- Place the syringe cover back on the unit.
Data Processing and Storage
The maximum number of data which can be stored in the DA-130N memory is 1,100 samples. Data can be set to be saved automatically or manually after each test. You can recall stored measurement data on the DA-130N display again or output the data to a printer or PC.
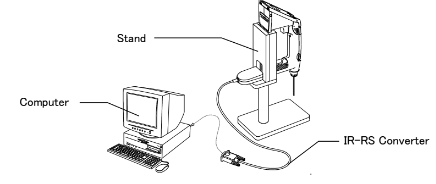
Exporting data to a PC via Infrared:
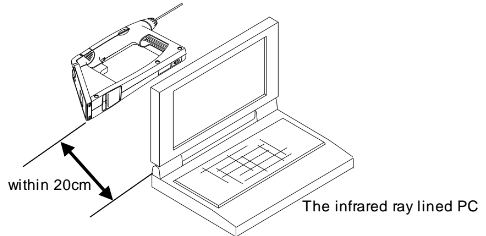
Exporting data to a PC via RS-232
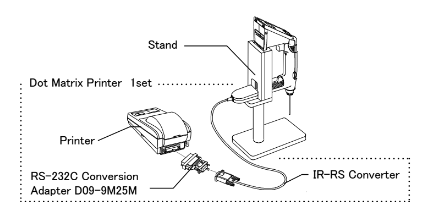
Sending data to a printer:
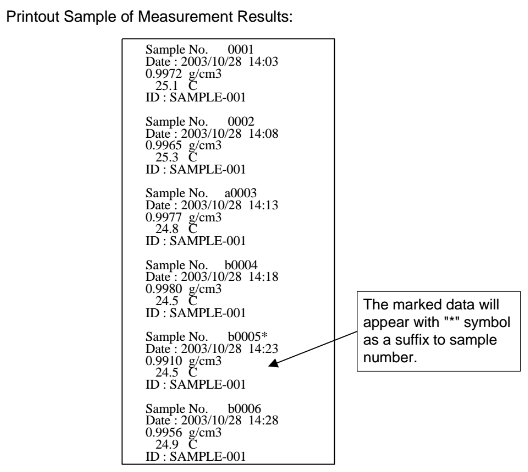
Example printout of data:
We hope this information about this particula density meter is helpful. If you need other capabilities beyond a traditional portable density meters refer to other bench top density meter models for increased accuracy.








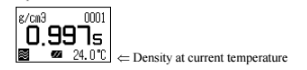
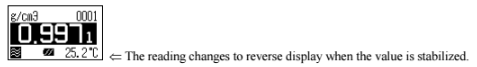











 A well-known company called Honeywell purchased and now controls the Hydranal line of Karl Fischer Reagents. You can still order Hydranal brand
A well-known company called Honeywell purchased and now controls the Hydranal line of Karl Fischer Reagents. You can still order Hydranal brand 

 Consistometer and are familiar with it's operation. Pressing down the gate and loading the trigger is step one. Pouring in your sample and scraping off the top to a clean and even surface is important to getting repeatable results. With a stopwatch in one hand, "popping" the trigger and letting the sample flow out and down the trough comes next. Some tests are designed to see how far a sample will flow in say, 10 seconds. Another testing approach might be to time the movement of the sample until it reaches a pre-determined point (bostwick). If you have ever noticed in the bottom of the tray there are lines with numbers ranging from 0 to 24 (you really can't see a number 24 because the tray stops exactly at what would be 24). Over the years these lines with numbers have affectionatly become to be known as "bostwicks".
Consistometer and are familiar with it's operation. Pressing down the gate and loading the trigger is step one. Pouring in your sample and scraping off the top to a clean and even surface is important to getting repeatable results. With a stopwatch in one hand, "popping" the trigger and letting the sample flow out and down the trough comes next. Some tests are designed to see how far a sample will flow in say, 10 seconds. Another testing approach might be to time the movement of the sample until it reaches a pre-determined point (bostwick). If you have ever noticed in the bottom of the tray there are lines with numbers ranging from 0 to 24 (you really can't see a number 24 because the tray stops exactly at what would be 24). Over the years these lines with numbers have affectionatly become to be known as "bostwicks". 

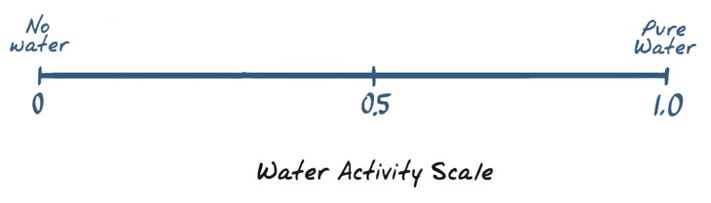
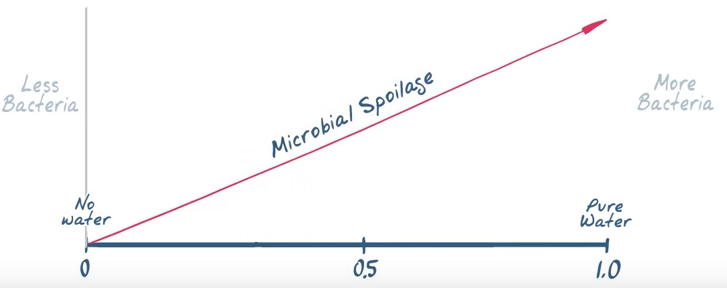
 thermodynamic measure of water expressed as the vapor pressure of water in a sample divided by vapor pressure of pure water at a given temperature.
thermodynamic measure of water expressed as the vapor pressure of water in a sample divided by vapor pressure of pure water at a given temperature.